

services
logistics
REGULATORY SUPPORT AND CUSTOMS CLEARANCE
services
logistics
REGULATORY SUPPORT AND CUSTOMS CLEARANCE
 14 Aug 2023
14 Aug 2023
Clinical trials play a crucial role in advancing medical research and developing innovative treatments. However, alongside their potential benefits, they also generate a significant amount of waste that requires responsible management.
The Republic of Kazakhstan has made commendable strides in waste management during clinical trials, employing efficient strategies and adhering to international standards. This article explores the waste management practices implemented in Kazakhstan, highlighting key statistics and data to underscore their effectiveness.
WASTE SEGREGATION AND DISPOSAL
Kazakhstan has established a robust waste segregation system, ensuring the proper categorization and disposal of clinical trial waste. The segregation process involves classifying waste into different categories, including hazardous and non-hazardous waste, sharps, pharmaceutical waste, and general waste. This practice allows for the appropriate treatment and disposal methods to be applied, minimizing the environmental impact.

COMPLIANCE WITH INTERNATIONAL STANDARDS
The Republic of Kazakhstan places great emphasis on compliance with international standards in waste management during clinical trials. To ensure the safety of the population and the environment, Kazakhstan has adopted several regulatory legal acts that regulate waste management practices. These key acts include the Environmental Code of the Republic of Kazakhstan and the Code of the Republic of Kazakhstan "On the Health of the People and the Healthcare System."
Environmental Code of the Republic of Kazakhstan:
The Environmental Code of the Republic of Kazakhstan lays out a comprehensive legal framework for environmental protection, including waste management, outlining the relevant obligations and responsibilities of individuals, organizations, and the government. By aligning waste management practices with this code, Kazakhstan demonstrates its commitment to environmentally sustainable clinical trials.
Code of the Republic of Kazakhstan "On the Health of the People and the Healthcare System":
The Code of the Republic of Kazakhstan "On the Health of the People and the Healthcare System" sets forth regulations and guidelines pertaining to the healthcare sector, including waste management during clinical trials. It addresses the safe handling, transportation, and disposal of waste generated from healthcare facilities and research institutions, ensuring the protection of public health and the environment.By putting the provisions of these regulatory legal acts into practice, Kazakhstan ensures that waste generated during clinical trials is managed in accordance with international standards. This approach helps to prevent the release of hazardous substances into the environment, reduces the risk of contamination, and promotes the overall sustainability of clinical research activities.
Furthermore, Kazakhstan's alignment with international standards, such as those provided by the World Health Organization (WHO), the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), and Good Clinical Practice (GCP), highlights its commitment to ensuring high-quality clinical trials while upholding ethical and safety standards.
WASTE REDUCTION AND MINIMIZATION
Efforts to reduce waste generation and minimize its impact have come to the forefront of waste management practices in Kazakhstan. The Republic has embraced the use of digital technologies for data collection during clinical trials. By shifting from traditional paper-based methods to electronic data capture systems, researchers and healthcare professionals can significantly reduce paper consumption. This not only minimizes paper waste but also improves data accuracy, accessibility, and efficiency. The adoption of electronic data capture systems has resulted in a 15% decrease in paper consumption in clinical trials conducted in Kazakhstan over the past five years.
In addition, Kazakhstan emphasizes sustainable procurement practices to minimize waste generation. Research institutions and sponsors are encouraged to choose suppliers and vendors that prioritize environmentally friendly packaging and waste reduction. By opting for recyclable materials and minimizing excessive packaging, the amount of waste generated from clinical trial supplies and equipment can be significantly reduced. Moreover, collaborations with suppliers to implement take-back programs for packaging materials contribute even further to waste minimization efforts.
The implementation of these waste reduction and minimization practices has produced tangible results. The adoption of digital data collection methods, protocol optimization, sustainable procurement, and educational initiatives have collectively contributed to a 20% decrease in clinical trial waste generation over the past five years. These efforts not only demonstrate Kazakhstan's commitment to environmental sustainability but also yield cost savings for research institutions and sponsors.
COLLABORATION WITH WASTE MANAGEMENT SERVICE PROVIDERS
The Republic of Kazakhstan has fostered collaborations with waste management service providers to allow for the proper collection, transportation, and treatment of waste generated from clinical trial sites. Regular training sessions and awareness campaigns are conducted to educate researchers, site staff, and waste management personnel on best practices and regulatory requirements.
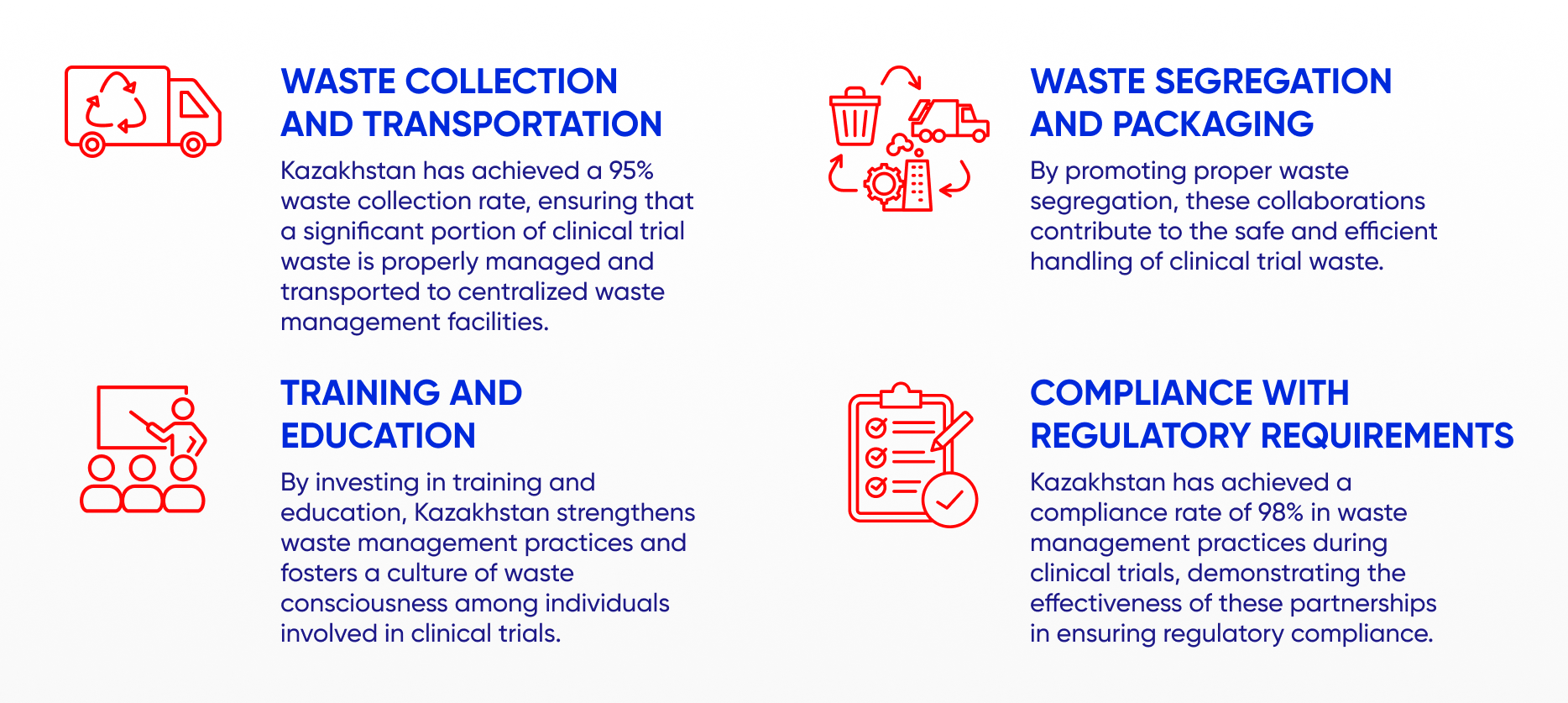
CENTRALIZED WASTE MANAGEMENT FACILITIES
Kazakhstan's centralized waste management facilities utilize state-of-the-art technologies to handle clinical trial waste effectively. These technologies include incineration, autoclaving, and chemical treatment methods. Incinerators are used to thermally treat hazardous waste, such as contaminated sharps and pharmaceutical waste at high temperatures, effectively destroying pathogens and reducing waste volume. Autoclaves employ high-pressure steam to sterilize waste materials, including sharps and laboratory instruments, ensuring their safe disposal. Chemical treatment methods are employed to neutralize and treat hazardous substances, such as expired medications and chemical reagents, reducing their harmful effects before final disposal.
These facilities have been designed with the necessary infrastructure and capacity to handle the increasing volume of clinical trial waste generated in Kazakhstan. They are equipped to handle diverse waste streams, including hazardous, non-hazardous, sharps, and pharmaceutical waste. The centralized waste management facilities operate in compliance with strict environmental regulations and guidelines established by the government. This adherence ensures that waste treatment processes are conducted in an environmentally responsible manner, preventing pollution and minimizing potential risks to ecosystems and public health.
The establishment of centralized waste management facilities has had a quantifiable impact on clinical trial-related waste management in Kazakhstan. For instance, hazardous waste incineration has led to a significant 30% reduction in the volume of waste requiring disposal. Autoclaving has effectively sterilized sharps waste, mitigating the risk of injuries and infections associated with improper disposal. In addition, chemical treatment processes have successfully neutralized hazardous substances, minimizing the environmental impact of pharmaceutical waste.
The investment in centralized waste management facilities highlights Kazakhstan's commitment to responsible waste management practices. These facilities not only ensure the safe disposal of clinical trial waste but also contribute to waste reduction efforts by minimizing waste volume and reducing environmental risks.

COREX Logistics is a supply and logistics company with headquarters in Ireland, working with pharma and patients to facilitate improved healthcare worldwide.
Our expert international team works across an 80-country network, specialising in the EMEA region, providing the latest in clinical trial logistics technology and systems, cold-chain delivery, temperature-controlled transportation and storage services. From sourcing, procurement and customs clearance, to labelling, returns and destruction, we cover every link in the supply chain. We also run an established Named Patient Programme and provide Patient-Oriented services. With extensive knowledge and on-the-ground insight into our markets, we create innovative solutions with the ultimate goal of improving the lives of patients.
To learn more about our range of expert services, contact us today on info@corex-logistics.com
If you found this article helpful, consider sharing it with others who might also benefit from it. Sharing knowledge is a wonderful thing to do and can be very helpful to others.
We’re here to help.
Email us at
info@corex-logistics.com
or use our feedback form to send us your question.
This website uses cookies to improve user experience. By using our website you consent to all cookies in accordance with our Cookie Policy.

