- about us
- services
services
- Clinical Trial Supply
- EARLY ACCESS PROGRAMS
- COREX SUPPORT SERVICES FOR RARE DISEASES
- Laboratory Kits Preparation
- Marking and labeling of medicinal products
- Return of medicinal products, materials and equipment
- Certified destruction of drugs
- Quarantine storage of drugs and clinical trial materials
logistics
- Temperature controlled transportation and storage services
- Patient Oriented Services
- Vaccine delivery and storage
- Cryogenic storage and transport
- COREX Logistics Courier service
- Direct-to-patient Delivery
- Global logistics for clinical trials
REGULATORY SUPPORT AND CUSTOMS CLEARANCE
- Customs clearance of medical cargo
- EXPERT IMPORTATION SOLUTIONS FOR LABORATORY EQUIPMENT AND MEDICAL PRODUCTS
- knowledge hub
- WhitePapers
- Media Center
- news
- videos
- events
- Tone Of Voice
- The Corporate Culture Guidebook
- case study
- contacts
- we're hiring
CLINICAL TRIAL LOGISTICS
REGULATORY SUPPORT
CLINICAL STUDIES SERVICES
- about us
- services
services
- Clinical Trial Supply
- EARLY ACCESS PROGRAMS
- COREX SUPPORT SERVICES FOR RARE DISEASES
- Laboratory Kits Preparation
- Marking and labeling of medicinal products
- Return of medicinal products, materials and equipment
- Certified destruction of drugs
- Quarantine storage of drugs and clinical trial materials
logistics
- Temperature controlled transportation and storage services
- Patient Oriented Services
- Vaccine delivery and storage
- Cryogenic storage and transport
- COREX Logistics Courier service
- Direct-to-patient Delivery
- Global logistics for clinical trials
REGULATORY SUPPORT AND CUSTOMS CLEARANCE
- Customs clearance of medical cargo
- EXPERT IMPORTATION SOLUTIONS FOR LABORATORY EQUIPMENT AND MEDICAL PRODUCTS
- knowledge hub
- WhitePapers
- Media Center
- news
- videos
- events
- Tone Of Voice
- The Corporate Culture Guidebook
- case study
- contacts
- we're hiring
- services
Back to the list 14 Jul 2020
14 Jul 2020
Clinical trials in Ukraine
Clinical trials are vital to the drug discovery process. They help to find effective drugs and treatments for diseases that affect the lives of millions of people all over the world. These treatments help to transform healthcare and save and improve the lives of people every day. Ukraine is an attractive emerging location for clinical trials. The country boasts several competitive advantages for sponsors seeking to test novel and innovative treatments including a large pool of qualified and GCP-trained medical personnel, well-equipped study sites, low costs relative to US and Western European countries, EU-harmonized standards, and large naïve populations (those who have not received treatments) in a wide range of therapeutic areas promising good recruitment potential. From 2015-2020, the number of clinical trials conducted in Ukraine has increased. These trials tested new treatments for many conditions including cancers, cardiovascular diseases, neurological conditions and diseases such as arthritis and endocrinological disorders. COREX Logistics supports clinical trials in Ukraine by delivering innovative and comprehensive clinical supply and logistics services.
How do clinical trials work?
Clinical trials are research studies performed in people to evaluate medical, surgical and behavioral interventions. They are the primary way that researchers can test if treatments, devices and other interventions are safe and effective. Other clinical trials are aimed at finding ways to detect diseases earlier while some are aimed at discovering ways to prevent diseases. Before clinical trials are approved to begin, researchers must test interventions in a laboratory setting and conduct studies on animals to test efficacy and safety. If these processes reveal favorable results the appropriate regulatory body will approve clinical trials in humans (e.g. FDA in US or EMA in EU).
Four Phases of Clinical Trials
Clinical trials proceed through four phases to test the effectiveness of a drug, find appropriate dosage and look for side effects.
- Phase I trial tests a drug usually on healthy volunteers (usually 20-80 people) to evaluate safety and look for side effects.
- Phase II normally involves more people (100-300). The emphasis in this second phase is to test effectiveness. This phase which can take several years aims to find out if a treatment works on people with a particular disease. Researchers continue to monitor safety and short-term side effects.
- Phase III tests the treatment on groups of people of different ages with different dosages and in combination with other drugs. This phase usually involves much larger numbers from several hundred to thousands of participants. At this stage, the decision is made to approve or deny registration and mass production of the drug.
- Phase IV trials take place after approval of a drug/treatment. Its safety and side effects are monitored in large diverse populations. Further post-registration studies help to identify the differences between this particular drug and its analogs by other manufacturers.
Clinical research in Ukraine: an emerging market
Ukraine is an emerging market for international sponsors conducting clinical trials. In recent years there has been steady growth in the number of clinical trials being conducted in Ukraine albeit from a very low base considering the size of the country and its population (41.98 million Eurostat, 2019). At present the leading therapeutic areas are oncology, neurology and psychiatry with dermatology and endocrinology also featuring strongly.
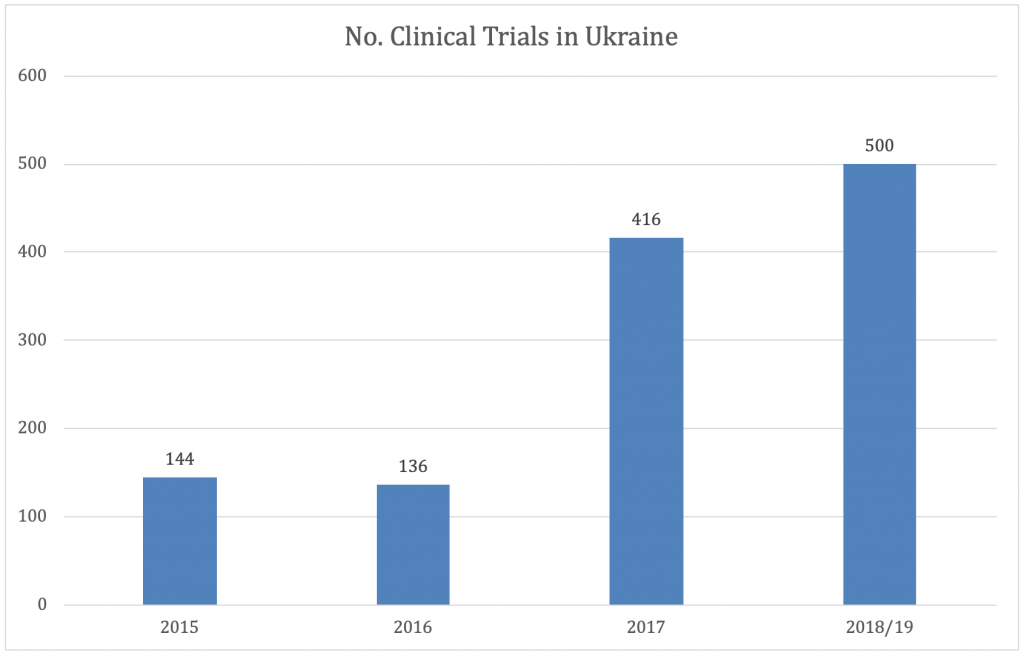
There is evidence that this upward trend is set to continue as sponsors begin to recognize the advantages of carrying out trials in Ukraine. Ukraine has a population of 41.98 million within which there are large naïve populations across a range of therapeutic areas making it a location with high recruitment potential for clinical trials. Physicians and patients may be favourably disposed to participating in clinical trials as a means of gaining access to novel treatments not available through the national health system. The country offers a large pool of motivated, qualified and GCP trained medical professionals and well-equipped medical facilities. In addition, in the last decade Ukraine has taken significant steps to harmonize its legislation and regulation regarding clinical research with the EU helping to reduce approval times and improve data quality. The Ukraine is a “priority partner” of the European Union meaning that it receives support from the EU to help reform its political system, ensure stability and promote economic growth. In 2014, Ukraine signed the Association Agreement (AA) with the EU, including the Deep and Comprehensive Free Trade Area (DCFTA) as a means of deepening ties. Ukraine has received more than €15billion in loans and grants to support its reforms. This alignment with the EU and commitment by authorities to reduce bureaucratic barriers bode well for further increases in the numbers of international clinical trials carried out in Ukraine.
Conditions for conducting research on humans in Ukraine
Any clinical trial can only be conducted with the informed consent of the patient. The tested drug in some situations can help people survive a disease or significantly improve their quality of life adding healthy years and helping them live normally. The conditions for the research including safety measures are outlined by the trial protocol. This protocol or plan for the clinical trial contains information about:
In Ukraine, clinical trials may only be carried out at approved Health Care Settings (HCS). In order to be approved, HCS must have a license for medical practice and a Minsitry of Health issued accreditation certificate. A Sponsor or CRO must obtain two documents to authorize clinical research. The first is an order from the Ministry of Health that approves the protocol of the trial. It is issued based on a positive conclusion from the State Expert Center. The other document is the conclusion of the local ethics committee of the medical institution where the trial will be carried out.
The patient's life and health are insured in accordance with the procedure stipulated by law. The procedures are provided exclusively by qualified personnel who are trained for this purpose. The high level of research conducted in Ukraine has been repeatedly confirmed by inspections of the European Medicines Agency and the US Food and Drug Administration.
Benefits for patients participating in clinical trials
There are many motivating factors which lead patients to participate in clinical trials. These include:
-
Clinical trials give the patient access to the latest drugs that cannot be purchased at the moment, especially for cancer treatment.
-
The patient is fully provided with additional drugs for basic therapy, which completely eliminates the need for payment from the test participant.
-
For many patients participating in clinical trials also gives them a way to participate more actively in their treatment.
-
Others say it is a way for them to help researchers find out more about their disease and be part of the process to provide better treatment options and find medical breakthroughs.
It is important to note that patients have a full right to withdraw from the research process at any stage. The agreement signed by the patient provides insurance for the duration of their participation in the procedures.
The future of clinical trials in Ukraine
As outlined above, clinical research is a promising emerging sector in Ukraine as the country has several competitive advantages that make it an attractive location for international sponsors. The benefits of further developing this sphere for the Ukraine include access for its patient population to expensive and innovative treatments not available through the health system. Participating in clinical trials also has advantages for physicians seeking to improve their patient outcomes and their experience with new interventions. Furthermore, developing clinical research and attracting international sponsors has significant economic benefits and will help educate and upskill health professionals in the country.
Ukraine has taken definite positive steps to increase its attractiveness as a location through economic and legislative reforms in the last decade. Further steps are needed to address some outstanding barriers including tackling issues around VAT on drugs and clinical supplies as well as continuing to reform its political and economic institutions in line with European standards and legislation. Reducing bureaucratic barriers for conducting research will help speed up approval processes and trial timelines. In addition, there is a need for greater emphasis on patient awareness and promotion of the benefits of clinical research to encourage greater participation. Recent steps include the establishment of a Ukrainian Association of Clinical Research, an NGO to promote the development of clinical research in the country. However, the future of the sector looks positive as several leading pharmaceutical companies have completed successful trials in recent years showing the potential of Ukraine as a key location for clinical research.
COREX Logistics your trusted partner for clinical trial supplies and logistics in Ukraine.
COREX Logistics is proud to provide tailored solutions that support clinical trials in Ukraine. Our expert team delivers innovative GxP compliant clinical supply and logistics services to pharmaceutical companies and CROs running trials in Ukraine. In 2019, COREX Logistics expanded its operations in the country by establishing a dedicated logistics hub in Kiev.
ABOUT US
COREX Logistics is a supply and logistics company with headquarters in Ireland, working with pharma and patients to facilitate improved healthcare worldwide.
Our expert international team works across an 80-country network, specialising in the EMEA region, providing the latest in clinical trial logistics technology and systems, cold-chain delivery, temperature-controlled transportation and storage services. From sourcing, procurement and customs clearance, to labelling, returns and destruction, we cover every link in the supply chain. We also run an established Named Patient Programme and provide Patient-Oriented services. With extensive knowledge and on-the-ground insight into our markets, we create innovative solutions with the ultimate goal of improving the lives of patients.
To learn more about our range of expert services, contact us today on info@corex-logistics.com
If you found this article helpful, consider sharing it with others who might also benefit from it. Sharing knowledge is a wonderful thing to do and can be very helpful to others.
CONTACT US
We’re here to help.
Email us at info@corex-logistics.com or use our feedback form to send us your question.This website uses cookies to improve user experience. By using our website you consent to all cookies in accordance with our Cookie Policy.
- services











